What if? <br>You could eliminate pre procedural steps for large bore vascular closure?, <br> You could heal and seal the arteriotomy from the inside?, <br> You could improve vascular complications?

What if <br>You could manage Large-Bore vascular closure without any Pre-procedurall steps?, <br> You could heal and seal the arteriotomy from the inside?, <br> You could improve vascular complications?

Clinical Need
Large arteriotomy management is a unique challenge.
A fully percutaneous approach has become the standard of care for most structural heart and aortic vascular procedures. There is increasing awareness that large catheter sizes can create unique challenges that require a dedicated closure solution.
Technology
Designed for
purpose
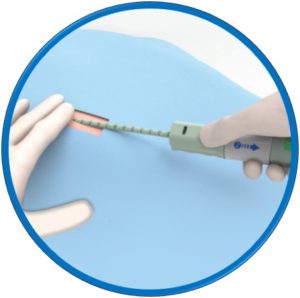
Suitable for arteriotomies up to 24F
No pre-procedural steps – OTW delivery
One device per arteriotomy
Fully synthetic absorbable implant
No sutures, no collagen, no metal
Simple and secure
device deployment
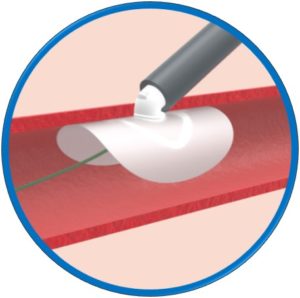
Automatic loading – Simple and intuitive delivery
Safety Guidewire remains in situ until implant release
Dedicated 0.035” compatible introducer
Patch based fully
absorbable implant

Ultra-low profile patch, rapidly endothelialised¹
Implant fully absorbed within 180 days¹
Abluminal surface matrix promotes adherence and healing¹
Implant fully absorbed within 180 days¹
Vessel remains patent - restored to its pre-procedure state

Implanted Patch
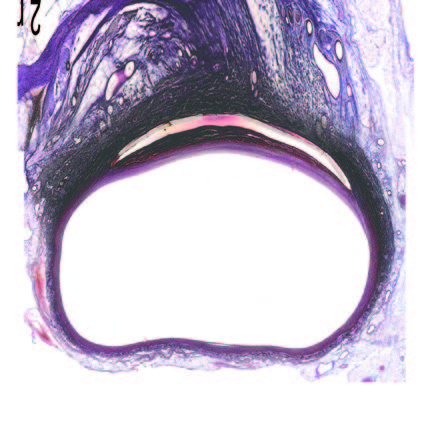
32
Days
Implant Fully Encapsulated
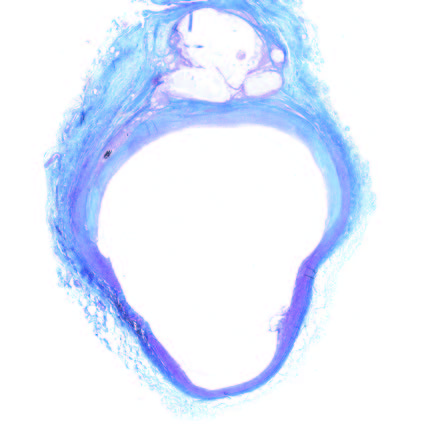
91
Days
Implant extra-vascular-vessel remodeling
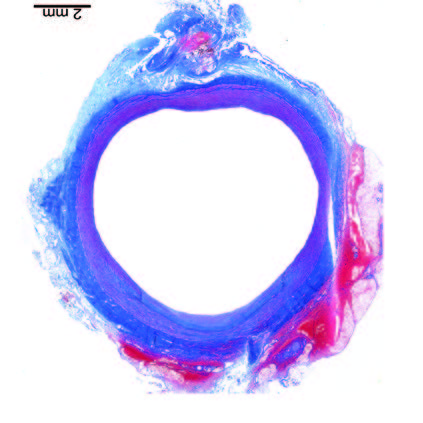
180
Days
Implant fully absorbed - vessel restored
Clinical Evidence
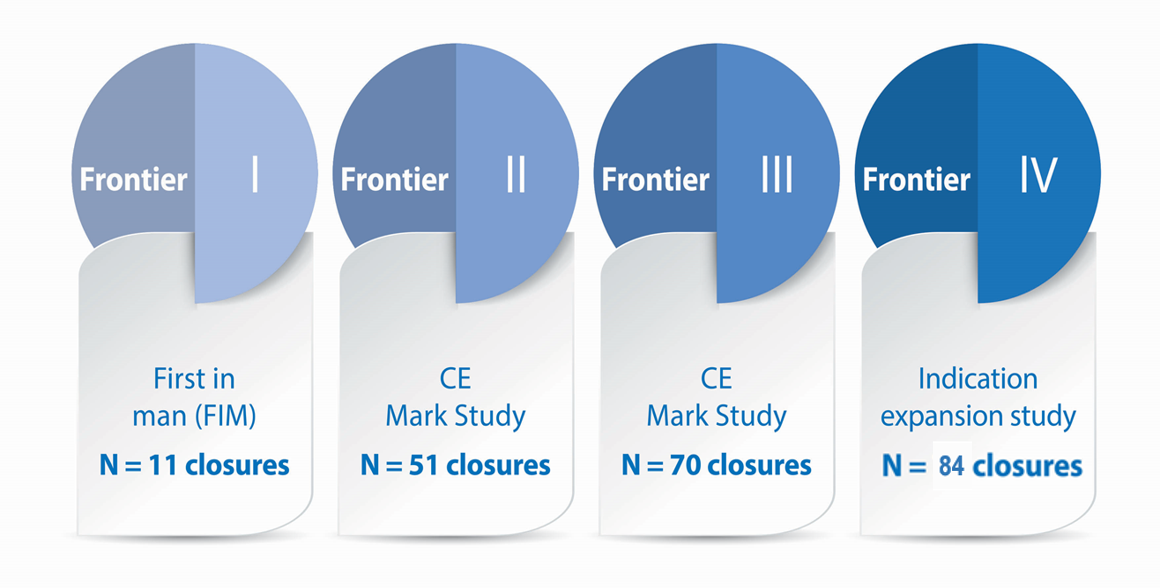
Frontier clinical programmes - An evolving database of safety and effectiveness
References:
1. Pre-clinical data – on file at Vivasure Medical
2. Clinical data Frontier I-IV studies on file at Vivasure Medical
About Us
Vivasure Medical Limited is a highly innovative medical device company based in the heart of Europe’s largest medtech hub in Galway, Ireland. The company develops advanced polymer implants and delivery systems, primarily focused on minimally invasive vessel closure in cardiology, interventional radiology and vascular surgery.
Established in 2009, Vivasure operates a fully integrated, ISO 13485 certified, R&D and manufacturing facility. The company is staffed by an experienced team of medical device professionals with expertise spanning research, engineering, material science, quality, regulatory, manufacturing, clinical and commercial roles.
Management team

Andrew Glass
Chief Executive Officer
Andrew has extensive leadership, commercial operations and product development experience in the medical technology and cardiovascular fields. Previous to Vivasure he held senior leadership roles at Abbott Laboratories for over fifteen years, most recently as regional director at Abbott Vascular where he was responsible for the commercial operations for thirteen European countries. Andrew also served in senior leadership positions in general management, business development, and marketing in Europe, Asia-Pacific, and the United States. Andrew has an MBA from the Harvard Business School and a BSE in Chemical Engineering from Princeton University.
Chief Executive Officer

Gerard Brett
Co-founder and Chief Operational Officer
Gerard has over 25 years international business management experience, 20 years in Medtech. Previously, he worked in the USA with Ciba-Geigy Corporation and with Boston Scientific in R&D, technology transfer and operations management. While with Boston, he was responsible for managing the transfer of over 25 vascular product lines into the Galway facility, and played a key role in the integration of the Schneider production into Galway. He co-founded Zerusa Limited, where as CEO he led the development and commercialisation of its Guardian® hemostasis technology in the EU and USA, prior to Zerusa’ s acquisition by Vascular Solutions.
Co-founder and Chief Operational Officer

Chris Martin
Co-founder and Chief Technical Officer
Chris has over 25 years of experience in the medical device industry – with 20 years in vessel closure, working with both start-up and multinational companies including American Cyanamid, David + Geck, Acuflex Microsurgical, Storz instruments, Johnson & Johnson, Nova Science and Zerusa. He has senior level experience in all aspects of research and product development, from concept to market, including: IP, development, pre-clinical, clinical, regulatory and production. He has tertiary qualifications in Engineering, Medicine and Biomedical Engineering. He is an inventor on 13 granted patents and over 30 applications worldwide.
Co-founder and Chief Technical Officer

David Keating
David is a Chartered Accountant with over 25 years financial management experience in both SMEs and large multinational companies having worked with KPMG, Grafton Group plc, Tomkins plc, Tesco Ireland, Uniphar plc, Greencore Group plc and Applegreen plc across a range of businesses sectors. David is a finance and business leader with extensive experience in corporate transactions including acquisitions, disposals, corporate restructuring and re-organisation as well as significant operational and financial experience. He recently held the positions of Interim Group Financial Controller in Greencore Group plc and Applegreen plc and was financial lead on a major financial restructuring, disposal and acquisition program with Uniphar plc.
Chief Financial Officer

Paul Geudens
Paul is a successful leader within the medical device industry having run sales and marketing organizations in both startup and Fortune 100 companies. Most recently, Paul led the market entry efforts for Neuravi Ltd until its acquisition by Johnson & Johnson, served as VP of Sales and Marketing for Stentys, and acted as Executive VP of Sales and Marketing for Surpass Medical. Prior to his startup endeavors, Paul spent 12 years with J&J in senior leadership positions across the company’s cardiovascular franchise.
Chief Commercial Officer

Kevin Timmins
Kevin has over 20 years of Quality and Regulatory experience within the Medical Devices industry. Having previously qualified as an Analytical Chemist, Kevin has worked in roles with increasing responsibilities in a number of multinational companies including Baxter, Boston Scientific, Stryker and Medtronic as well as Irish companies including Creganna during which time he was certified as a Project Management Professional (PMP). Prior to joining Vivasure, Kevin has been responsible for the initial certification and/or upgrade of a number of quality systems and was the Quality lead on multiple Class II and III medical device projects including vascular and cardiovascular stents and delivery systems as well as aortic and pulmonic structural heart products from the design phase through worldwide commercial approval.
VP Regulatory and Quality Affairs

Mark McGoldrick
Mark has over 25 years of R&D experience in the pharmaceutical and medical device industries. He has worked in roles covering a wide spectrum of responsibilities in a number of multinational and start-up companies including Elan, Pharmaplaz, Boston Scientific, and Innocoll. Qualifying as an analytical chemist and holding an MSc and MBA, Mark has led teams and successfully delivered technologies including Class II and Class III medical devices from concept through clinical trials and into commercial release. Mark has authored and co-authored 14 patents for implantable, bioabsorbable devices.
Director Research and Development
Board of Directors

Bernard Collins
As the chairman and an independent non-executive director of Vivasure Medical, Dr Bernard Collins brings a wealth of business experience. He serves on the Board of Directors of several U.S. and Irish life-science companies. Prior to forming Lifemed, his consultancy company, Dr Collins served for 10 years as Vice President of International Operations at Boston Scientific Corporation and before this held senior executive positions in life-science companies including Baxter Corporation. His general management experience in the medical device and health care industry spans start-ups, turnarounds, leveraged buyouts and IPOs for both large and small businesses. Dr Collins holds a BA Honors in Applied Industrial Psychology/Business from University College Cork, Ireland and an honorary Doctorate in Law from the National University of Ireland Galway. He is a recipient of the prestigious RDS Gold Medal for Industry in recognition of his extraordinary contribution to the development of the biomedical industry in Ireland.
|
Chairman

Gerard Brett
Co-founder
Gerard has over 25 years international business management experience, 20 years in Medtech. Previously, he worked in the USA with Ciba-Geigy Corporation and with Boston Scientific in R&D, technology transfer and operations management. While with Boston, he was responsible for managing the transfer of over 25 vascular product lines into the Galway facility, and played a key role in the integration of the Schneider production into Galway. He co-founded Zerusa Limited, where as CEO he led the development and commercialisation of its Guardian® hemostasis technology in the EU and USA, prior to Zerusa’ s acquisition by Vascular Solutions.

Barbara Castellano
Barbara Castellano is a partner in Panakès Partners. She has more than 20 years of experience in Medtech, in particular in Cardiovascular, having held corporate senior positions at Sorin (now Livanova) and CID Cardiovascular (now Alvimedica). By leading strategic marketing, business development, clinical, quality and regulatory departments, Barbara developed and brought innovative cardiovascular technologies to the market. More recently, as CEO of the advisory firm TechWald SpA, she has developed strong experience in the Medtech startup world, successfully raising over €15m in venture capital funding for innovative European and Israeli medical technology companies. Barbara holds a degree in Medicinal Chemistry and an Executive MBA (both magna cum laude) from SDA Bocconi in Milan.
|

Robert (Chip) Hance
Robert (Chip) Hance is the former CEO of Creganna Medical, an Ireland-based medical device company with over 2,000 employees worldwide that was acquired by TE Connectivity in 2016. In 2012-13, Chip was an Entrepreneur-in-Residence at the Center for Devices and Radiological Health within the United States Food and Drug Administration (FDA), where he co-led the Innovation Pathway team focused on streamlining aspects of medical device clinical trials in the U.S. Prior to his FDA experience, Chip was most recently President of Abbott Vascular, the cardiovascular device division of Abbott. Over a decade in interventional cardiology at Abbott Vascular, he led the organization to global leadership in the drug-eluting stent market through the launch of Xience in the U.S., Japan and China. Chip also led the development and CE marking of Absorb, a bioabsorbable scaffold for the treatment of coronary disease and the acquisition and international expansion of Mitraclip, a percutaneous mitral valve repair device. Chip earned a bachelor’s degree in Chemical Engineering from the Massachusetts Institute of Technology and a master’s degree in Business Administration from Harvard Business School
|

Justin Lynch
Justin Lynch is a partner in Fountain Healthcare Partners and also serves as the CFO of the firm. He joined the team prior to the launch of Fund I in 2008. Justin has a BA in Accounting & Finance from Dublin City University, an MBA from Trinity College Dublin and is a member of the Chartered Institute of Management Accountants. He has over 25 years of experience in capital markets, corporate finance, entrepreneurial and venture investing across multiple sectors, the latter half in life science. Justin has a particular interest in the medical device sector and led the firm’s investments in Vivasure Medical and in Neuravi where he also serves as a board member.

Anne Portwich
Anne Portwich, PhD, is a Partner of LSP, an independent European investment firm, providing financing for private and public life sciences and healthcare companies. With over €2 billion under management and offices in Amsterdam, Munich and Boston, LSP is one of Europe’s leading life sciences investor. Anne currently serves on the supervisory boards of iSTAR Medical, OneProjects, Atlantic Therapeutics, MedEye, ViCentra and Vivasure Medical. Previous board positions include Sapiens Steering Brain Stimulation (acquired by Medtronic in 2014), ActoGeniX (acquired by Intrexon in 2015), Nexstim (IPO in 2014) and Neuravi (acquired by Codman Neuro in 2017). Prior to joining LSP, Anne was a scientist and project leader at OctoPlus, a Dutch company offering advanced research services to pharmaceutical and biotechnology companies. She obtained an MSc in biochemistry from the University of Hanover and received her PhD from the Max Planck-Society.
|

Mark Redshaw
Mark Redshaw is an Investment Director at Evonik Venture Capital. Where he focuses on deals relating to nutrition, health and care, and specialty additives. Mark holds board seats at a number of portfolio companies. Prior to joining the team founding Evonik Venture Capital in 2012, Mark held positions within the Nutrition & Care business of Evonik. This included time responsible for sustainability, heading the Evonik’s global Animal Nutrition Services and running the operative business in Africa and the Middle East. Mark studied agriculture at the University of Reading and received a PhD from the University of Nottingham.

David Hochman
David Hochman has served as Chairman and Chief Executive Officer of Orchestra BioMed since May 2018. From 2006 to 2019, he was Managing Partner of Orchestra Medical Ventures, a medical technology venture capital firm. He also served as President of Accelerated Technologies, Inc., a medical device accelerator company managed by Orchestra. David has over 23 years of healthcare entrepreneurial, venture capital and investment banking experience. He is also Chairman of the Board of Motus GI (NASDAQ: MOTS). He was a co-founder and served as a board member of Corbus Pharmaceuticals Holdings, Inc. (NASDAQ: CRBP), a clinical stage biopharmaceutical company, from 2013 to 2020. Prior to joining Orchestra Medical Ventures, Mr. Hochman was Chief Executive Officer of Spencer Trask Edison Partners, LLC, an investment partnership focused on early stage healthcare companies. He was also Managing Director of Spencer Trask Ventures, Inc. during which time he led financing transactions for over twenty early-stage companies raising over $420 million. From 1999 to 2006, Mr. Hochman was a board advisor of Health Dialog Services Corporation, a leader in collaborative healthcare management that was acquired in 2008 by the British United Provident Association for $750 million. From 2005 to 2007, he was a co-founder and board member of PROLOR Biotech, Inc., a biopharmaceutical company developing longer lasting versions of approved therapeutic proteins, which was purchased by Opko Health (NYSE: OPK) in 2013 for over $600 million. He currently serves as President of the Board of the Mollie Parnis Livingston Foundation. He has a B.A. degree with honors from the University of Michigan.
|
Board Observer
Our Partners








News

Vivasure Medical Begins Clinical Evaluation of Next-Generation PerQseal+ Device
20 April 2021 – GALWAY, Ireland – Vivasure Medical® today announced the first patient was enrolled in the Frontier V study, a European multicenter study

Vivasure Medical Announces Andrew Glass as Chief Executive Officer
6 April 2021 – GALWAY, Ireland – Vivasure Medical® today announced Andrew Glass has assumed the role of Chief Executive Officer. Founder and former CEO

Vivasure Medical co-sponsors Learning Center session at LINC 2021
18 January 2021 – GALWAY, Ireland – Vivasure Medical® will co-sponsor a session focused on endovascular innovation at the Leipzig Interventional Course 2021 (25-29 January
Phone or Fax
Tel: +353 91 395 440
Fax: +353 91 395 399
Address
Parkmore Business Park West
Galway
Ireland
H91 V3KP
