
Vivasure Medical Begins Clinical Evaluation of Next-Generation PerQseal+ Device
20 April 2021 – GALWAY, Ireland – Vivasure Medical® today announced the first patient was enrolled in the Frontier V study, a European multicenter study


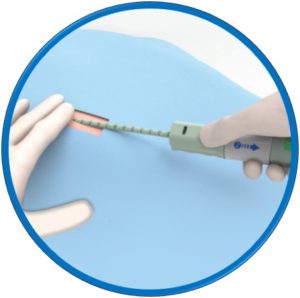
Suitable for arteriotomies up to 24F
No pre-procedural steps – OTW delivery
One device per arteriotomy
Fully synthetic absorbable implant
No sutures, no collagen, no metal
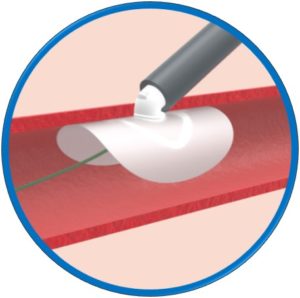
Automatic loading – Simple and intuitive delivery
Safety Guidewire remains in situ until implant release
Dedicated 0.035” compatible introducer

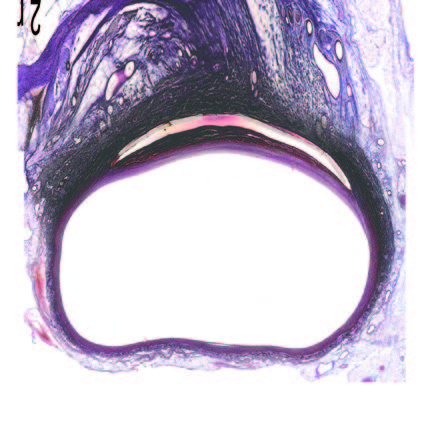
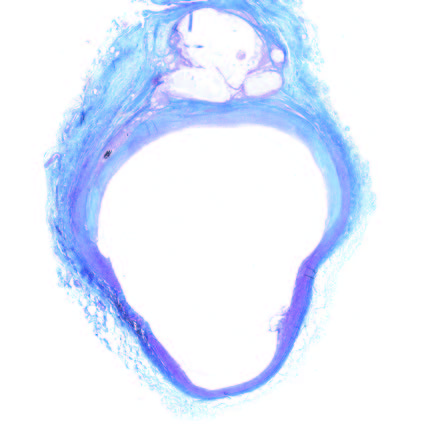
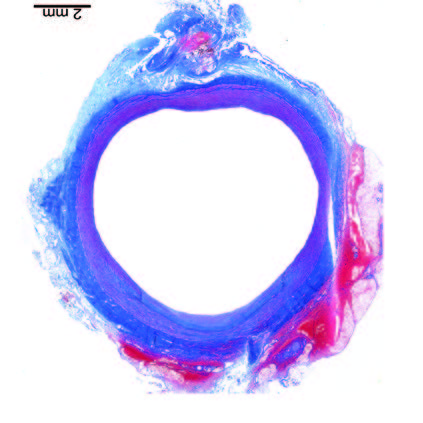
Ultra-low profile patch, rapidly endothelialised¹
Implant fully absorbed within 180 days¹
Abluminal surface matrix promotes adherence and healing¹

References:
1. Pre-clinical data – on file at Vivasure Medical
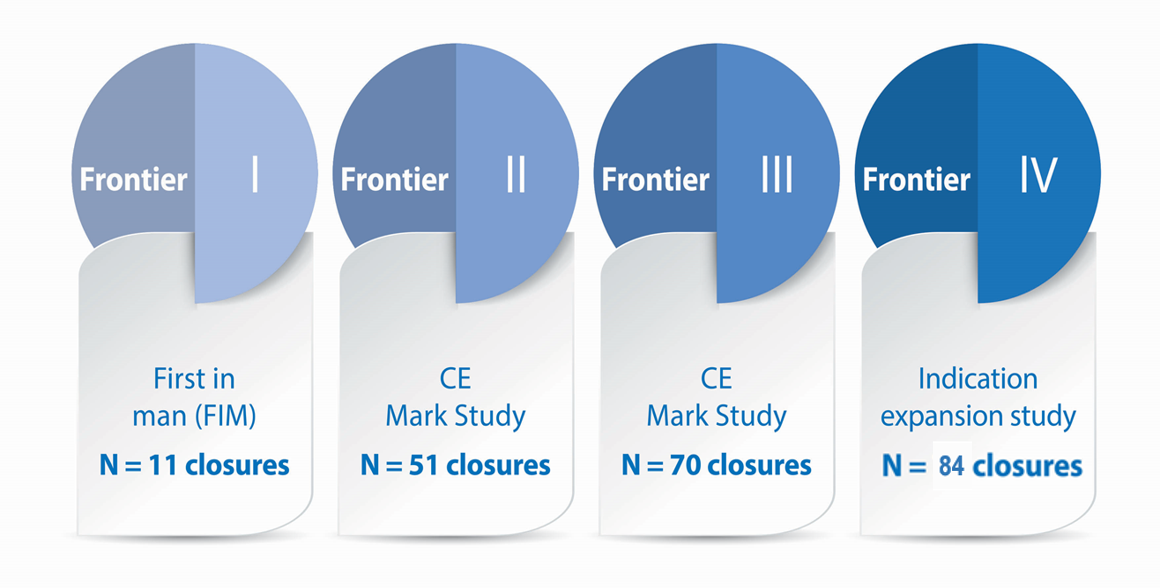
2. Clinical data Frontier I-IV studies on file at Vivasure Medical
Vivasure Medical Limited is a highly innovative medical device company based in the heart of Europe’s largest medtech hub in Galway, Ireland. The company develops advanced polymer implants and delivery systems, primarily focused on minimally invasive vessel closure in cardiology, interventional radiology and vascular surgery.
Established in 2009, Vivasure operates a fully integrated, ISO 13485 certified, R&D and manufacturing facility. The company is staffed by an experienced team of medical device professionals with expertise spanning research, engineering, material science, quality, regulatory, manufacturing, clinical and commercial roles.

Chief Executive Officer

Co-founder and Chief Operational Officer

Co-founder and Chief Technical Officer

Chief Financial Officer

Director Research and Development

Chairman




Board Observer

Board Observer









20 April 2021 – GALWAY, Ireland – Vivasure Medical® today announced the first patient was enrolled in the Frontier V study, a European multicenter study

6 April 2021 – GALWAY, Ireland – Vivasure Medical® today announced Andrew Glass has assumed the role of Chief Executive Officer. Founder and former CEO

18 January 2021 – GALWAY, Ireland – Vivasure Medical® will co-sponsor a session focused on endovascular innovation at the Leipzig Interventional Course 2021 (25-29 January
Tel: +353 91 395 440
Fax: +353 91 395 399
Parkmore Business Park West
Galway
Ireland
H91 V3KP
Copyright © 2020-2023 Vivasure Medical Ltd. All rights reserved. Terms and conditions PerQseal is a registered trademark of Vivasure Medical Ltd. | Privacy Statement